SmartGstore
Duhaney Clinical Trials Investment Dashboard
Duhaney Clinical Trials Investment Dashboard
Couldn't load pickup availability
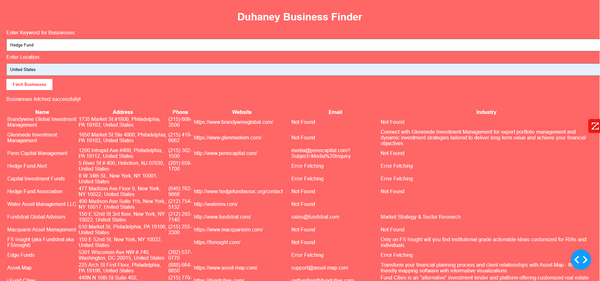
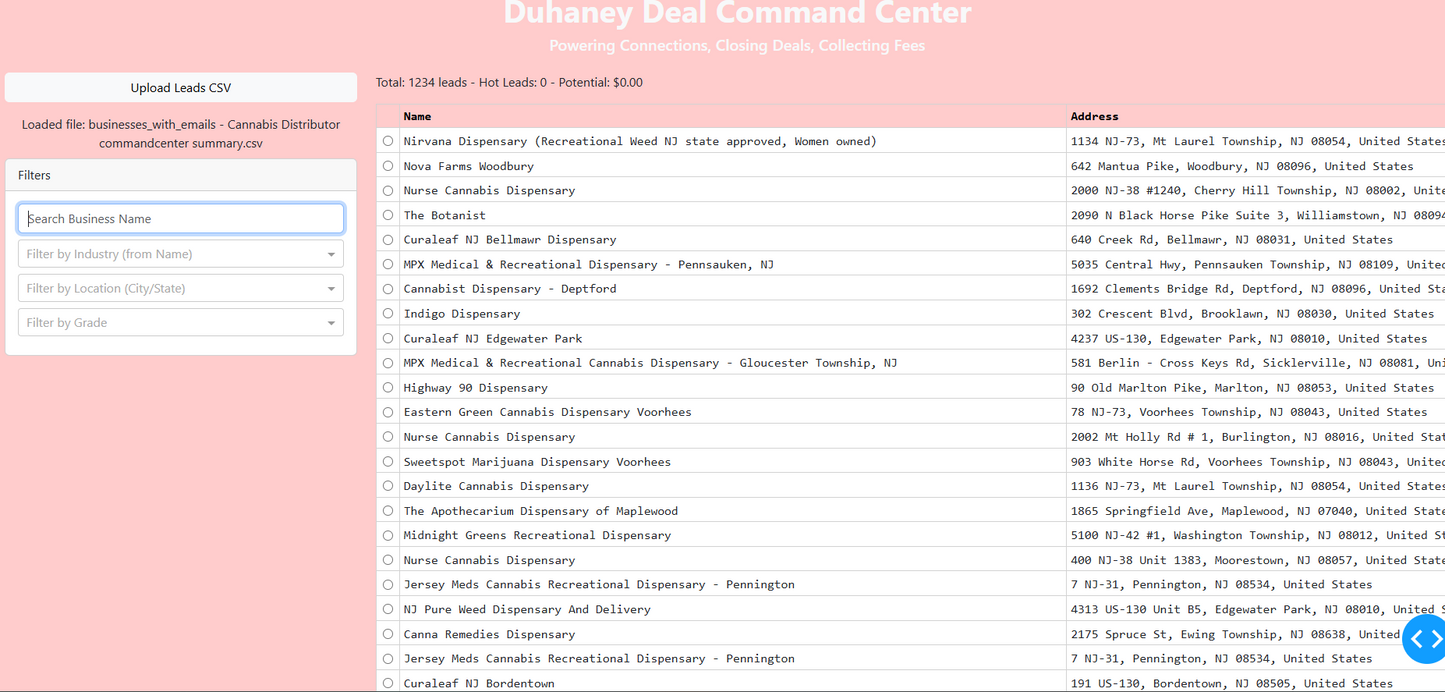
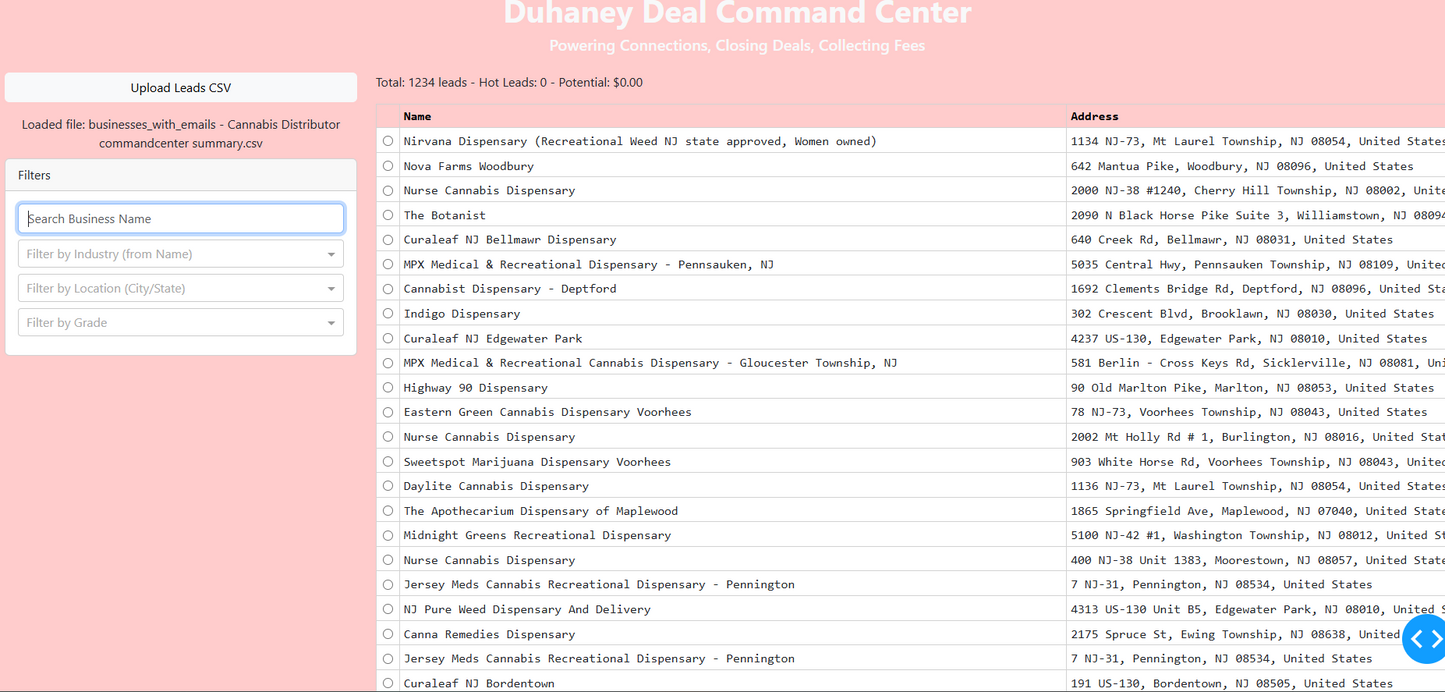
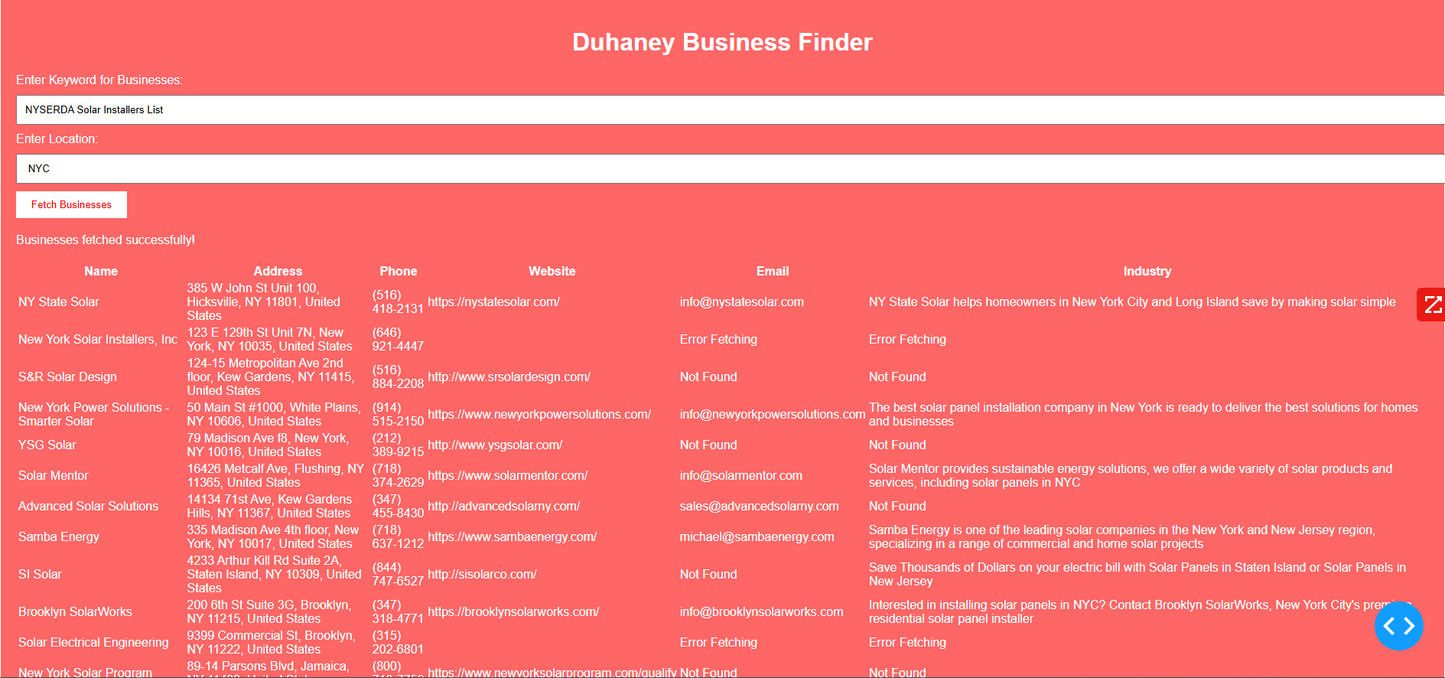
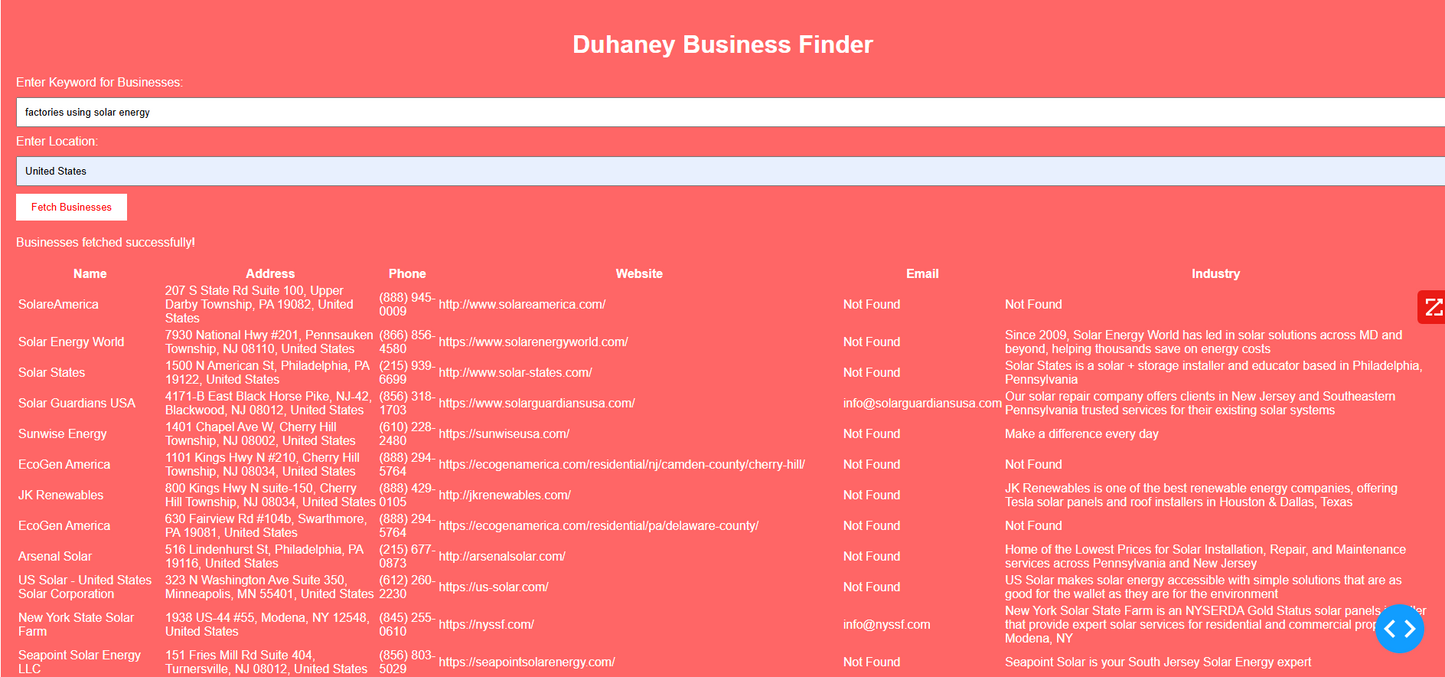
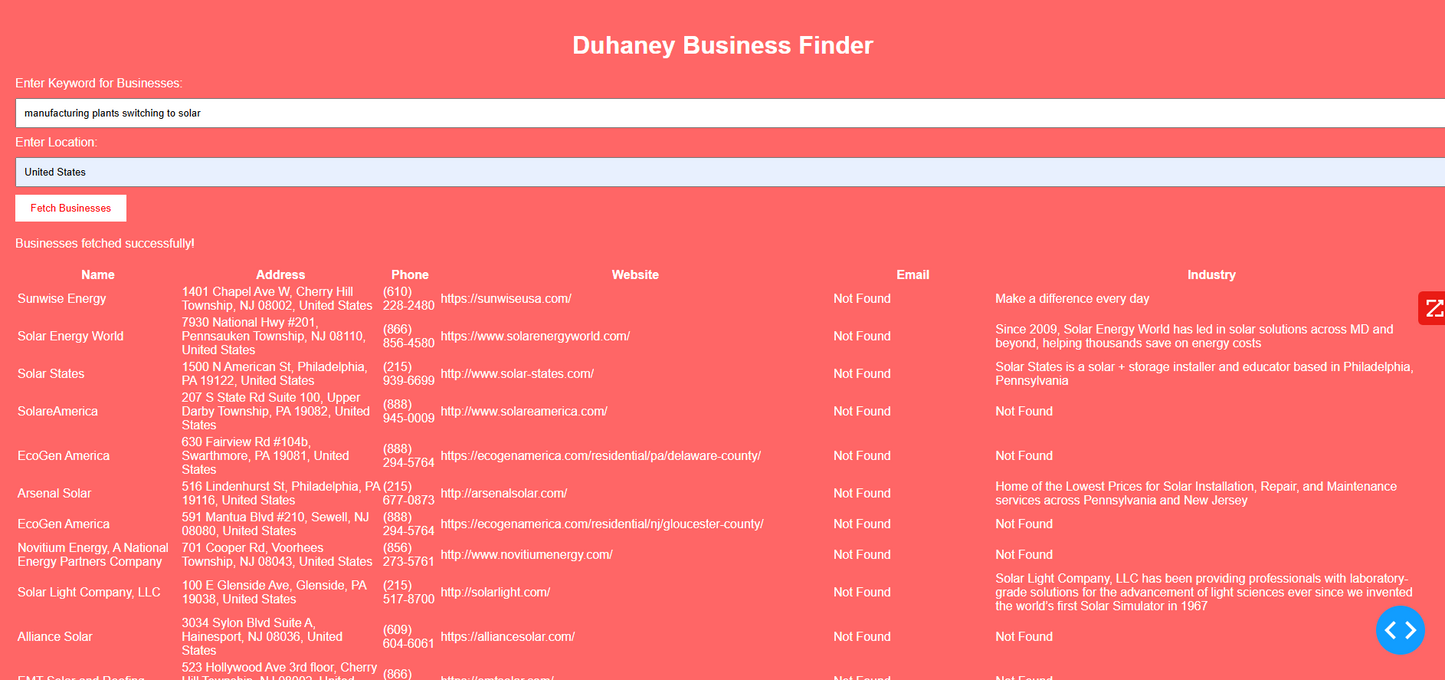
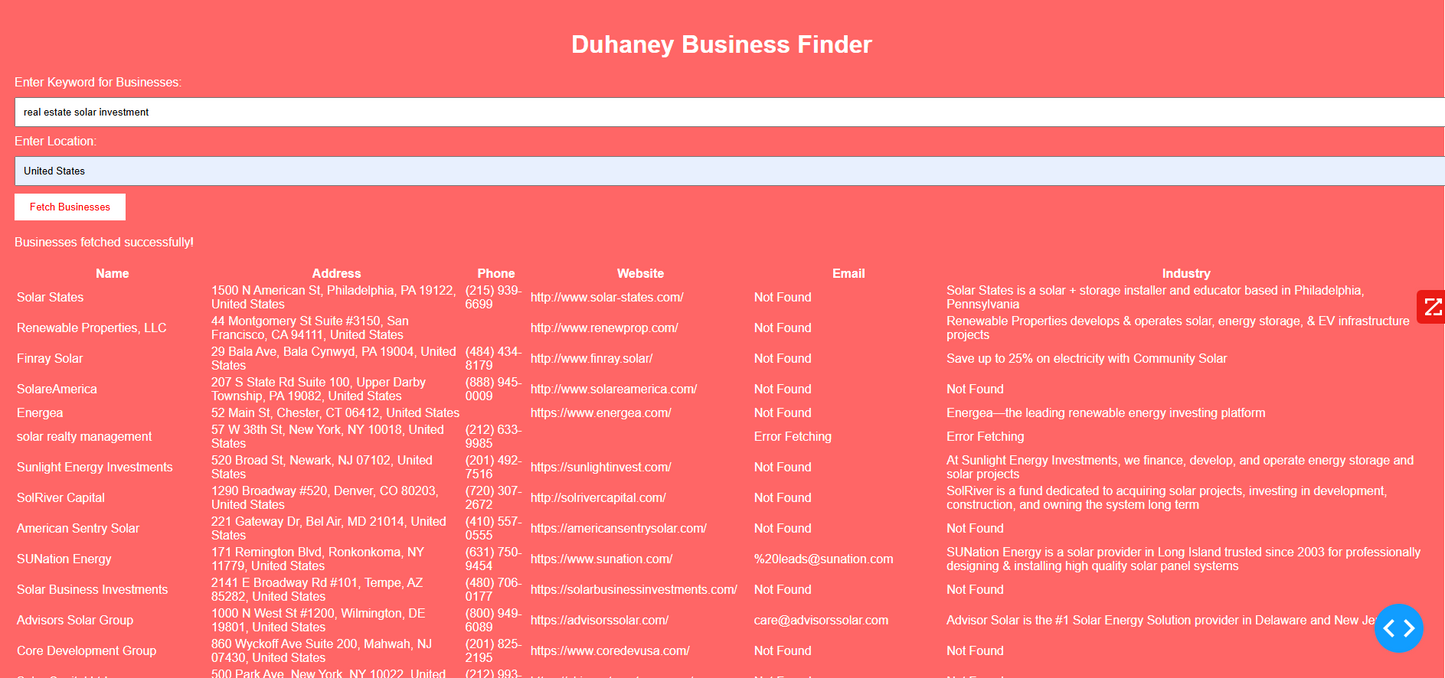
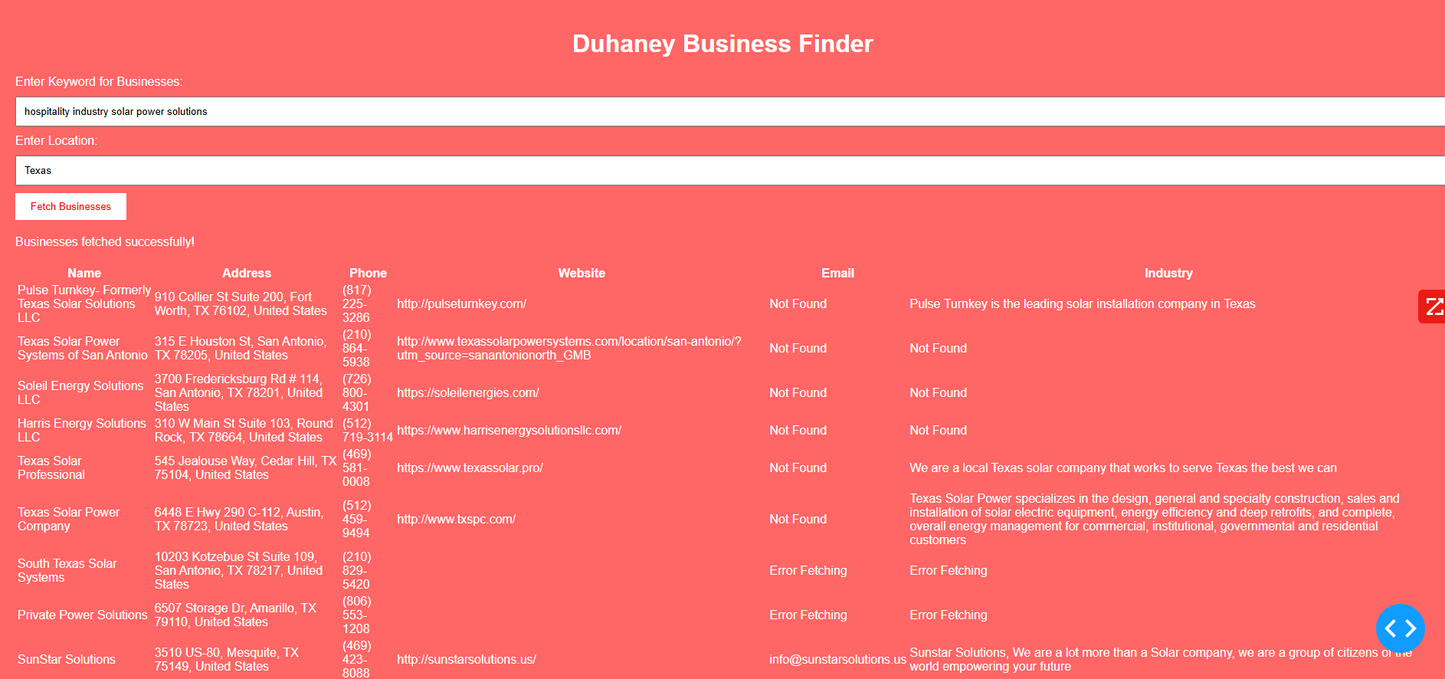
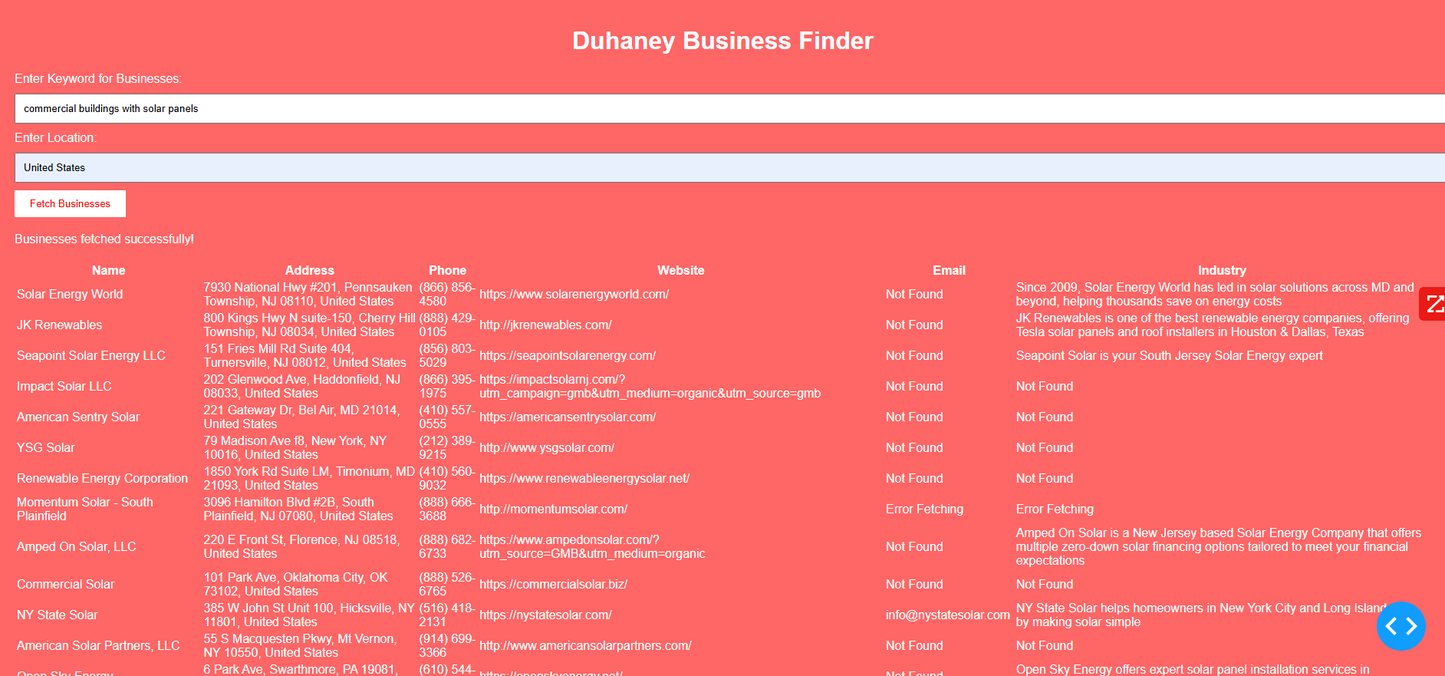
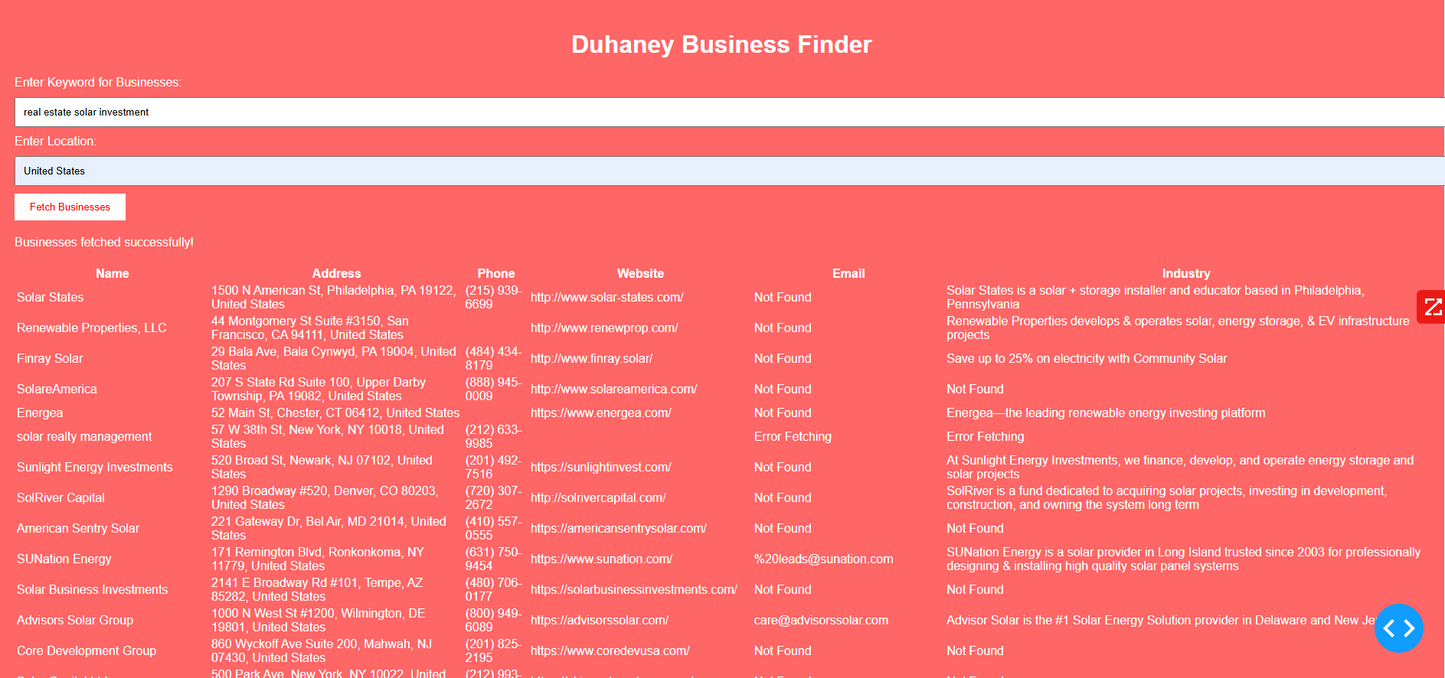
Duhaney Real Estate Deal Command Center – Full Ecosystem
Duhaney Clinical Trials Investment Dashboard – Your Insider Guide to Drug Success & ROI
Starting Price: $1500.00 USD
The Truth About Clinical Trials Nobody Tells You
If you’ve ever tried to predict which drug will win FDA approval, or which trial will attract the next big investment, you already know the truth — **the data is scattered, outdated, and full of noise**.
Most clinical reports tell you **what happened**, but they never tell you **what it really means for your investment, your startup, or your next move**.
---
Let’s Deframe That Entire Process
Why guess when you could have a **real-time dashboard** that connects the dots between:
- ✅ Which sponsors dominate in each therapeutic area
- ✅ The **real cost per patient** by condition and phase
- ✅ The historical **ROI for every major trial category**
- ✅ Which drugs have **the highest probability of approval**
- ✅ And exactly which conditions are pulling the most investment dollars right now
---
Now Let’s Reframe — What You’re Actually Getting
The **Duhaney Clinical Trials Investment Dashboard** isn’t a static report — it’s your **decision engine**. Every time you need to answer questions like:
- 💰 Which drug is about to make investors rich?
- 📊 Which sponsor should my startup partner with?
- ⚕️ Which condition is about to have a blockbuster approval?
- 📈 What’s the **per-patient cost vs revenue potential** for each drug?
- 🔍 Which trials are actually worth tracking — and which are just noise?
…you load the dashboard and the answers are right there.
---
What’s Inside Your Personalized Dashboard
✅ Pre-loaded with the **top 50 therapeutic areas** (Sickle Cell, Oncology, Rare Diseases, and more) ✅ Full **financial modeling** — cost per patient, projected revenue per patient, and calculated ROI ✅ FDA study phases & statuses — see which trials are closest to approval ✅ Sponsor and CRO performance tracking — know who delivers and who burns money ✅ Real-time NCT Number cross-referencing — no more disconnected data
---
Why This Dashboard is Different
✅ **Built directly from FDA clinicaltrials.gov data** — no second-hand garbage ✅ **Includes historical AND current data** — so you can track trends, not just snapshots ✅ **Custom-built to your needs** — you tell us your focus (condition, phase, region, sponsor type) and we personalize the dashboard to fit you.
---
Call Before Purchase – Here’s Why
Because this isn’t a one-size-fits-all product. Every investor, startup, or pharma exec has a unique focus — oncology vs rare disease, early-stage vs late-stage trials, US vs global markets.
That’s why you must call me directly before purchase, so I can personally customize your dashboard to fit your strategy.
Contact Rohan Duhaney
Phone: (856) 522-3601
Email: smartduhaney@gmail.com
---
Future Price Increases
The current price of **$1500.00 USD** reflects the early release version. As new data sources, advanced modeling, and predictive AI get added, the price will increase significantly.
**Secure your personalized dashboard now at today’s rate — and lock in free updates for the first 60 days.**
---
Ready to See What Real Clinical Intelligence Feels Like?
Call me today — and let’s build your **custom intelligence engine** so you can make smarter, faster decisions in clinical development and biotech investing.
Rohan Duhaney
Phone: 856-522-3601
Email: smartduhaney@gmail.com
Website: smartduhaney.com
PROOF OF WORK
Healthcare Solutions Dashboard Output
Brought to you by Rohan Duhaney
Contact: 856-522-3601 | Email: smartduhaney@gmail.com | Website: smartduhaney.com
----------------------------------------------------------------------
Condition: Sickle Cell Disease
Cure: DRUG: ATG|DRUG: fludarabine|DRUG: cyclophosphamide|RADIATION: Total body irradiation|PROCEDURE: Stem cell infusion|DRUG: Sirolimus|DRUG: mycophenolate mofetil
For Investors only investing in a study:
Financial Forecast:
• Total Cost per Patient: $250,000.00
• Total Revenue per Patient: $2,100,000.00
• ROI: 740.0%
NCT Number: NCT03121001
Study Phase: PHASE2
Study Status: RECRUITING
Additional Insights:
• Devices/Interventions: No device listed
• Enrollment: 50
• Sponsor: University of Illinois at Chicago
----------------------------------------------------------------------
Healthcare Solutions Dashboard Output
Brought to you by Rohan Duhaney
Contact: 856-522-3601 | Email: smartduhaney@gmail.com | Website: smartduhaney.com
----------------------------------------------------------------------
Condition: HbS Disease|Hemoglobin S Disease|Sickle Cell Anemia|Sickle Cell Disorders|Sickling Disorder Due to Hemoglobin S|Sickle Cell Disease
Cure: OTHER: TCRα/β+ and CD19+ depleted haploidentical stem cell transplantation|OTHER: Matched sibling donor transplantation
For Investors only investing in a study:
Financial Forecast:
• Total Cost per Patient: $1,060,000.00
• Total Revenue per Patient: $8,904,000.00
• ROI: 740.0%
NCT Number: NCT04201210
Study Phase: PHASE2
Study Status: RECRUITING
Additional Insights:
• Devices/Interventions: No device listed
• Enrollment: 212
• Sponsor: University of Regensburg
----------------------------------------------------------------------
Healthcare Solutions Dashboard Output
Brought to you by Rohan Duhaney
Contact: 856-522-3601 | Email: smartduhaney@gmail.com | Website: smartduhaney.com
----------------------------------------------------------------------
Condition: Sickle Cell Disease|Diffuse Myocardial Fibrosis
Cure: DRUG: Losartan
For Investors only investing in a study:
Financial Forecast:
• Total Cost per Patient: $120,000.00
• Total Revenue per Patient: $1,008,000.00
• ROI: 740.0%
NCT Number: NCT05012631
Study Phase: PHASE2
Study Status: RECRUITING
Additional Insights:
• Devices/Interventions: No device listed
• Enrollment: 24
• Sponsor: Children's Hospital Medical Center, Cincinnati
----------------------------------------------------------------------
We HAVE UNLIMITED INFORMATION READY TO EXPLORE